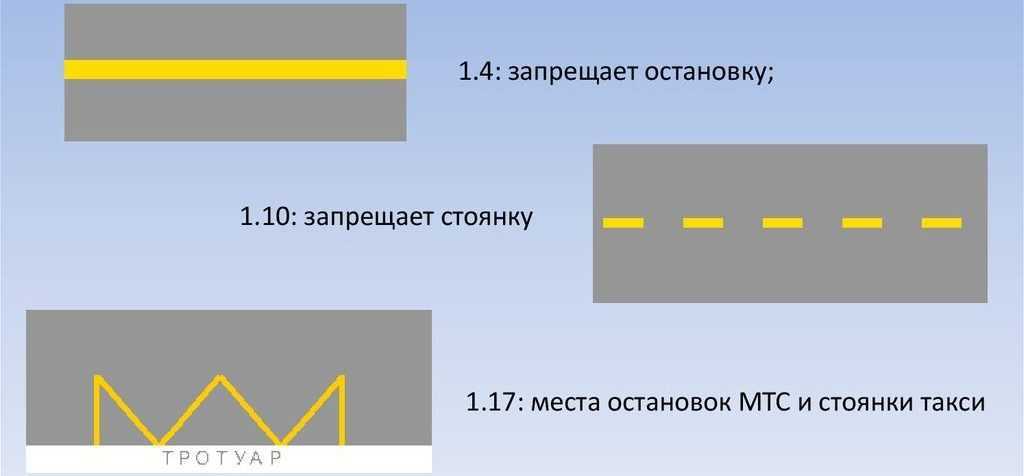
28.04.2018 года в Москве изменена разметка перекрестков. Вместо традиционных горизонтальных белых полос, использованы желтые
Описываем распространённые признаки износа колодок модели Рено Логан. Рассказываем, как подготовить автомобиль и выполнить
Найденные результаты для как поменять на приоре ромашки видео.
Замена ремня Время идет Калин Ремень ГРМ Лада Калина это кольцевой ремень из резины,
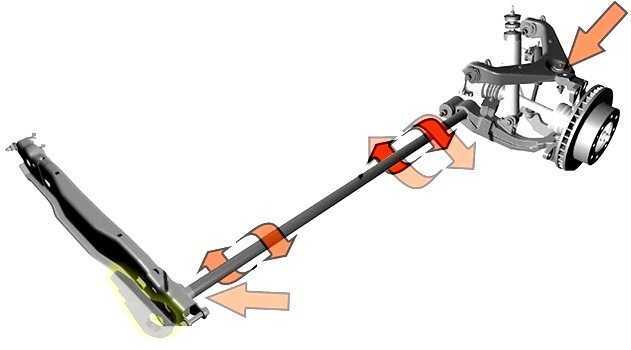
Как устроена подвеска на автомобиле - особенность конструкции и принцип действия. Расскажем, как диагностировать
Интервал замены масла в двигателе, в каком порядке производить замену и как сделать это
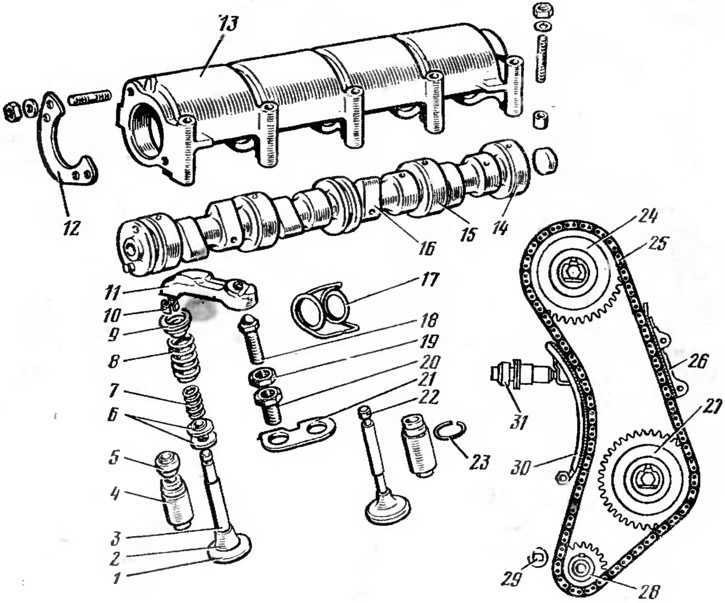
Как выставить метки на ВАЗ 2106, ГРМ 2103. Электронное зажигание на ВАЗ 2106: схема,
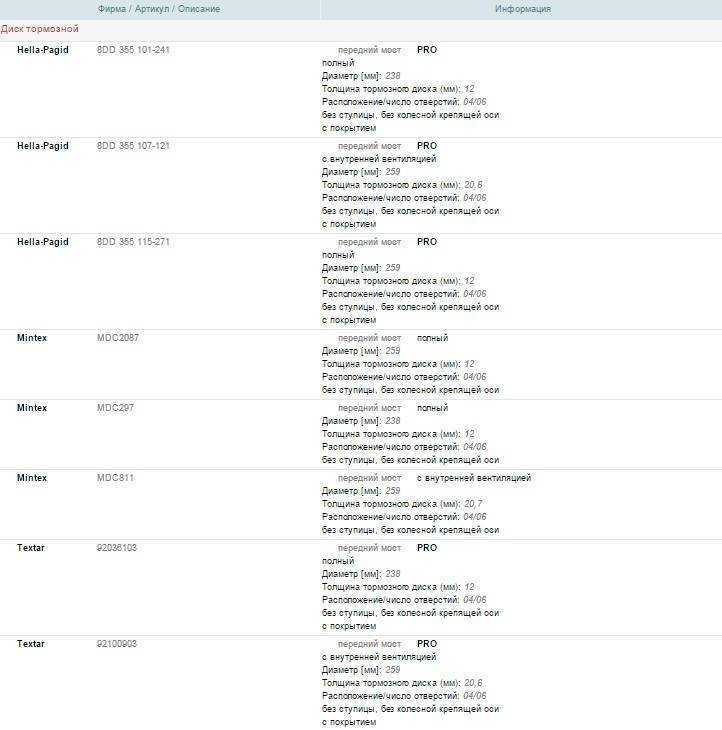
Рабочий тормозной цилиндр встречается не на всех автомобилях. Его замену или ремонт часто производят
Вязкостная муфта в системе охлаждения двигателя автомобиля применяется в качестве альтернативы электрическому вентилятору. Рассмотрим,
В ходе эксплуатации автомобиля его детали постепенно изнашиваются, резиновые изделия стареют, что приводит к
В чем причина длительного запуска двигателя из-за долгой прокрутки стартером, как будто сел аккумулятор.
Если вас интересует двигатель ваз 2107 - какие двигатели ставят, модификации вы можете найти
При покупке Ниссан Х Трейл возникает вопрос: какую КПП лучше взять? Все за и
Как узнать владельца по номеру автомобиля через интернет бесплатно. База данных ГИБДД, онлайн-сервисы, VIN-код
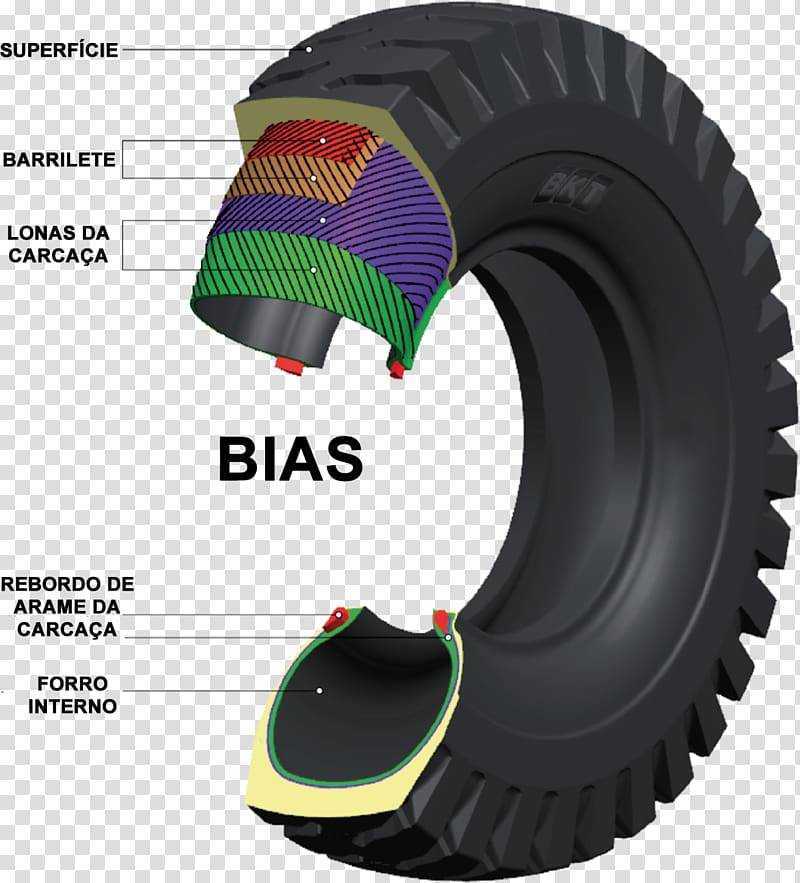
В процессе движения автомобиля шина подвергается постоянному износу. Износ шины сказывается ее эксплуатационных показателях,
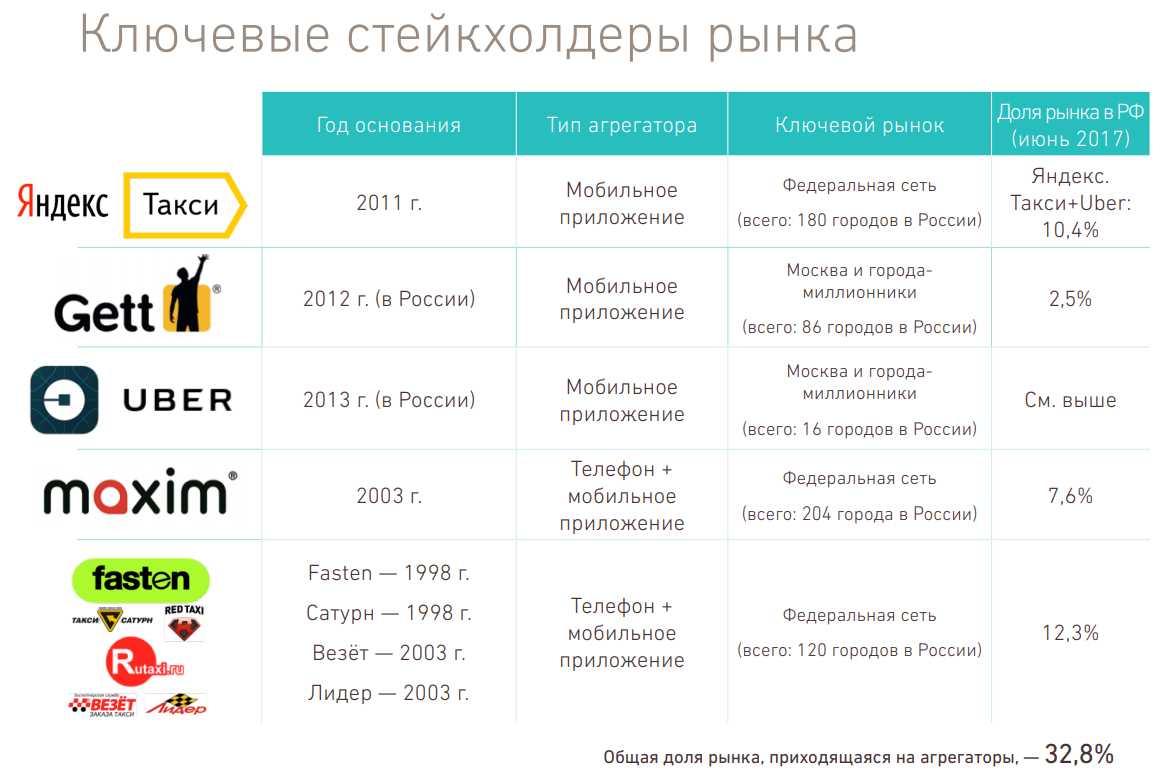
Тарифы Uber-такси действующие с 1 января 2019 года в Москве, Санкт-Петербурге и других крупных
Как правильно ухаживать за турбиной дизельного двигателя. Основные причины поломки турбины. Основные шаги ремонта
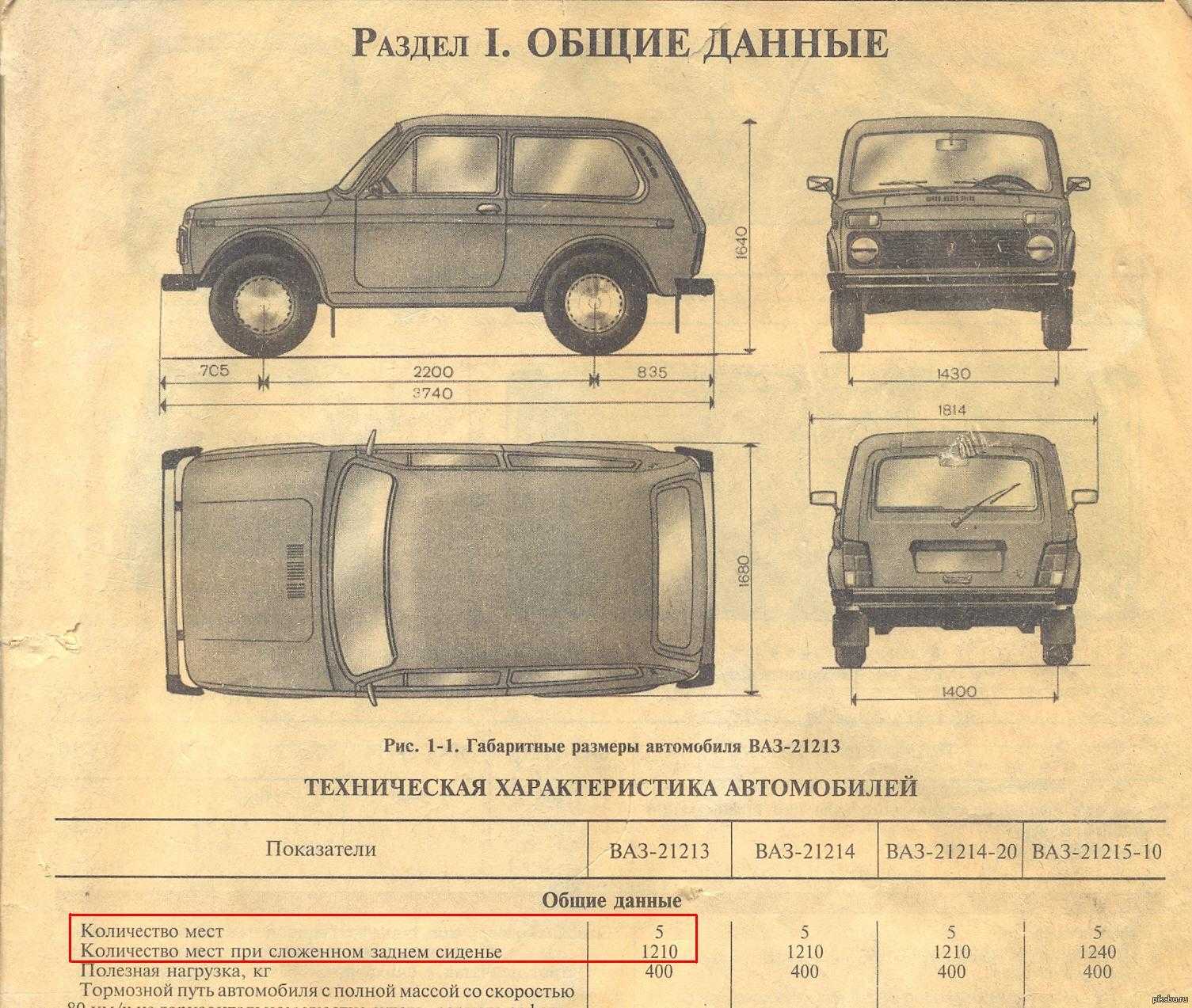
Обзор и характеристики отечественного автомобиля ВАЗ 2104. Фото, видеоматериалы, тюнинг и мнение экспертов об
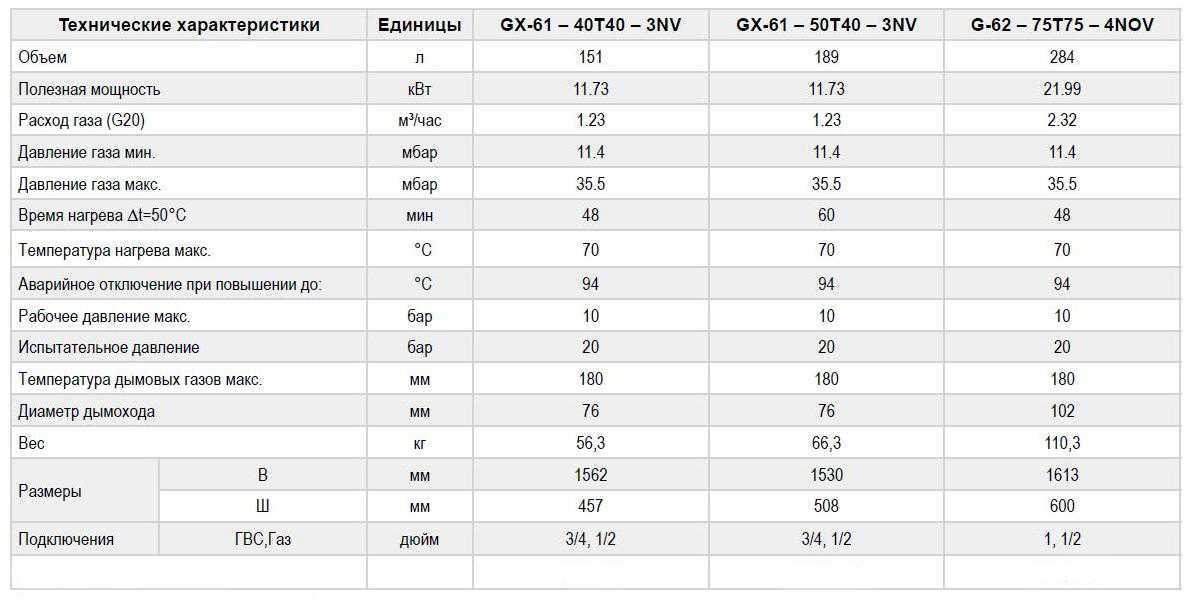
Тракторы Т-40М, Т-40АМ, Т-40АНМ. Техническое описание и инструкция по эксплуатации, масса, двигатель. Модификации Т-40
Торсионная подвеска принцип работы, секреты конструкции. Особенность популярности такого рода подвесок заключается в его
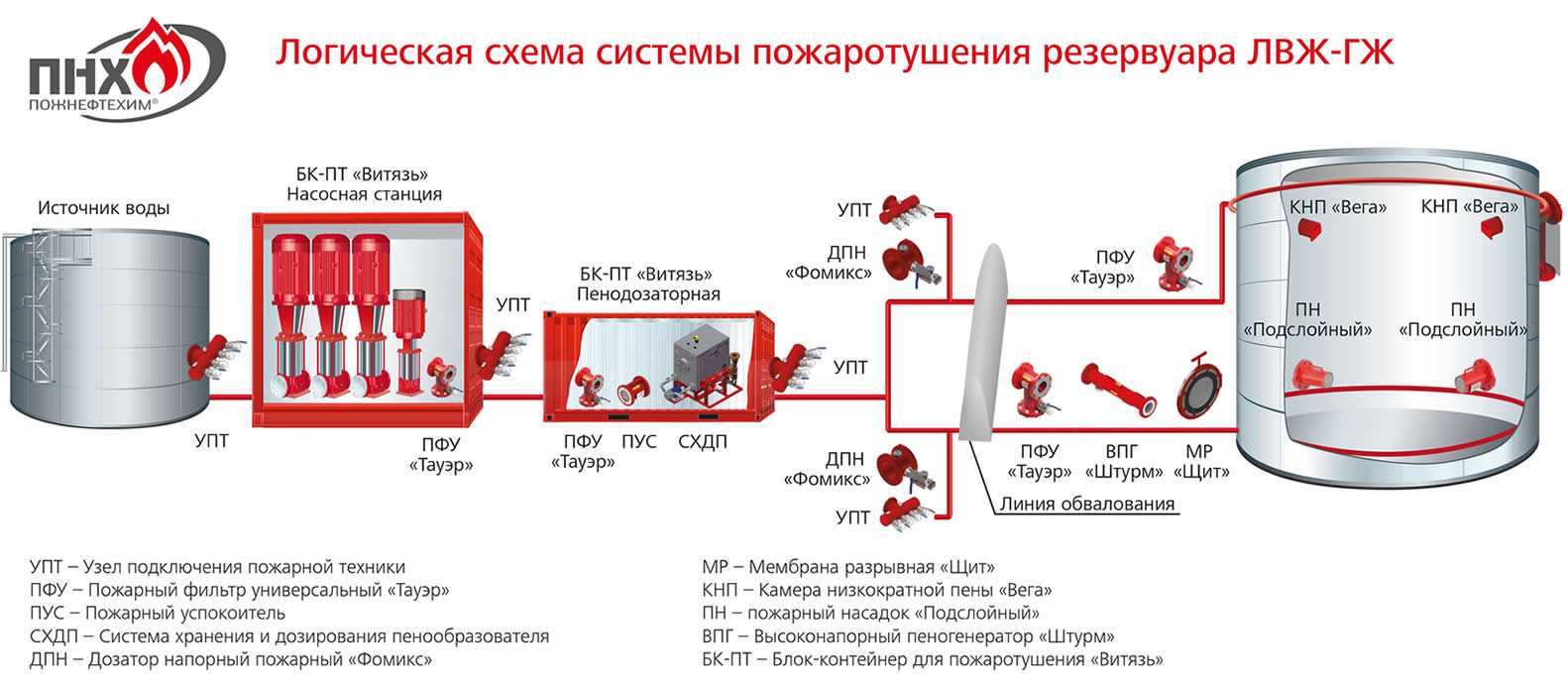
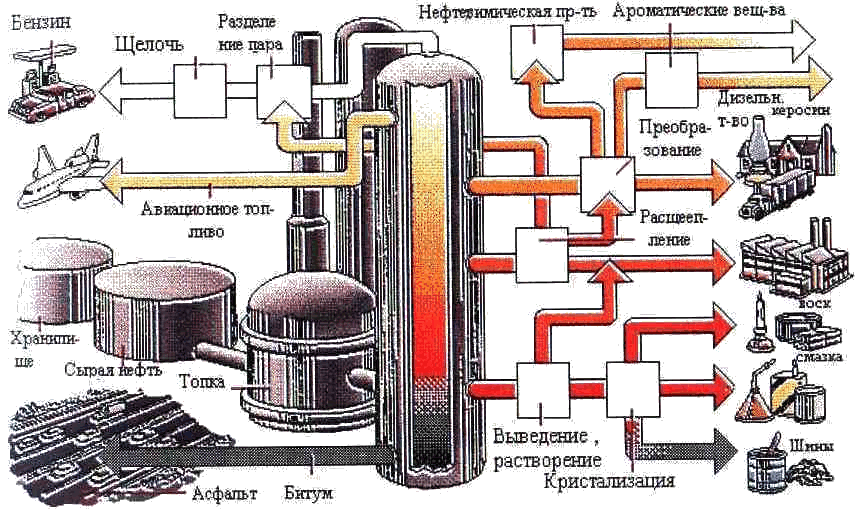
Вещества выделяемые при горения нефти, нефтепродуктов и класс пожаров. Пожарная опасность при добыче, переработки.
На что смотреть при покупке бу Фольксваген Пассат? Узнай подробнее о проблемах и неисправностях
Тахометр на Ардуино и датчике Холла для станка с ЧПУ. Собираем своими руками -